Feature: We Unpack The COVID-19 Vaccine Trial Numbers, Theatre Style!
Unsure about how safe the COVID-19 vaccines are? Here is an explainer using theatre venue capacities and more to help make sense of the numbers
of the Pfizer/BioNTech COVID vaccine
The first COVID-19 vaccine was approved for use in the UK on 2 December. It was developed by two pharmaceutical companies, Pfizer and BioNTech. Initial doses are being given to the older and more vulnerable in our society, including some famous faces like Sir Ian McKellen!
Drug development normally takes a really long time, at least a decade. The idea of a vaccine for a new disease being developed in just under a year is understandably something to be cautious about.
I hope this article can bring some reassurance to you that these vaccines are a well-tested, safe and effective way for us to be able to return to our favourite venues.
Much as I'd love to spend all my time writing about theatre, my main day job actually involves writing about new medicines, and I have a PhD in drug development. It's not often I get to combine my science and theatre worlds.
I thought it might be helpful to outline the numbers we have so far from the leading vaccine clinical trials using theatre venues to understand better how many people were used in vaccine tests. For an overview of how the Pfizer/BioNTech vaccine works, check out this video.
Here is a very brief introduction to the different types of clinical trial, through the lens of the development of a new play or musical:
Phase 1
The medicine under investigation is tested in a small number of healthy volunteers (20 to 100, think of the Menier Chocolate Factory) to see if there are any unsavoury effects.
Think of this as a table reading for a new play. No sets, no choreography, no costumes, or direction just yet, just the bare bones of a script to see if the structure and flow of the show works.
Phase 2

The drug in question is tested in a slightly larger group of patients (100 to 500, think of both spaces in The Other Palace combined) to see if it has its desired effect.
If it's a diabetes treatment, for example, you would now test it in patients with diabetes.
This stage is a bit like a workshop. Actors are given some direction, but a show still has a long way to go before a proper run in a larger theatre.
Phase 3
This is the last stage a treatment needs to pass before being approved on the drug market. The medication is tested in an even larger number of patients (usually 300 to 3000, think of the massive Edinburgh Playhouse).
This is like an "out of town" run of a large-scale show, where the show is staged as fully as possible to prove it is received well by a live audience before, say, transferring to the West End or Broadway.
Phase 4
Once a medicine has been approved, the investigations don't stop there! Scientists need to keep an eye on medications as more and more people take a particular treatment in the real-world versus the more controlled clinical trial setting.
This is a bit like a musical supervisor such as Alex Lacamoire coming in to watch a performance of Dear Evan Hansen every few months to check that everything and everyone in the show is up to scratch.
Now, let's look at the Pfizer/BioNTech COVID-19 vaccine in more detail...
Pfizer/BioNTech Vaccine Effectiveness vs the Royal Albert Hall
The Pfizer/BioNTech mRNA vaccine was approved in the UK on 2 December. The results for the Phase 3 trial were published as an independently-reviewed research article on 10 December.
This information was fed gradually to the UK drug regulators over the past few months to help speed up the paperwork. No steps were skipped in the process; there was just a lot less waiting time between those steps.
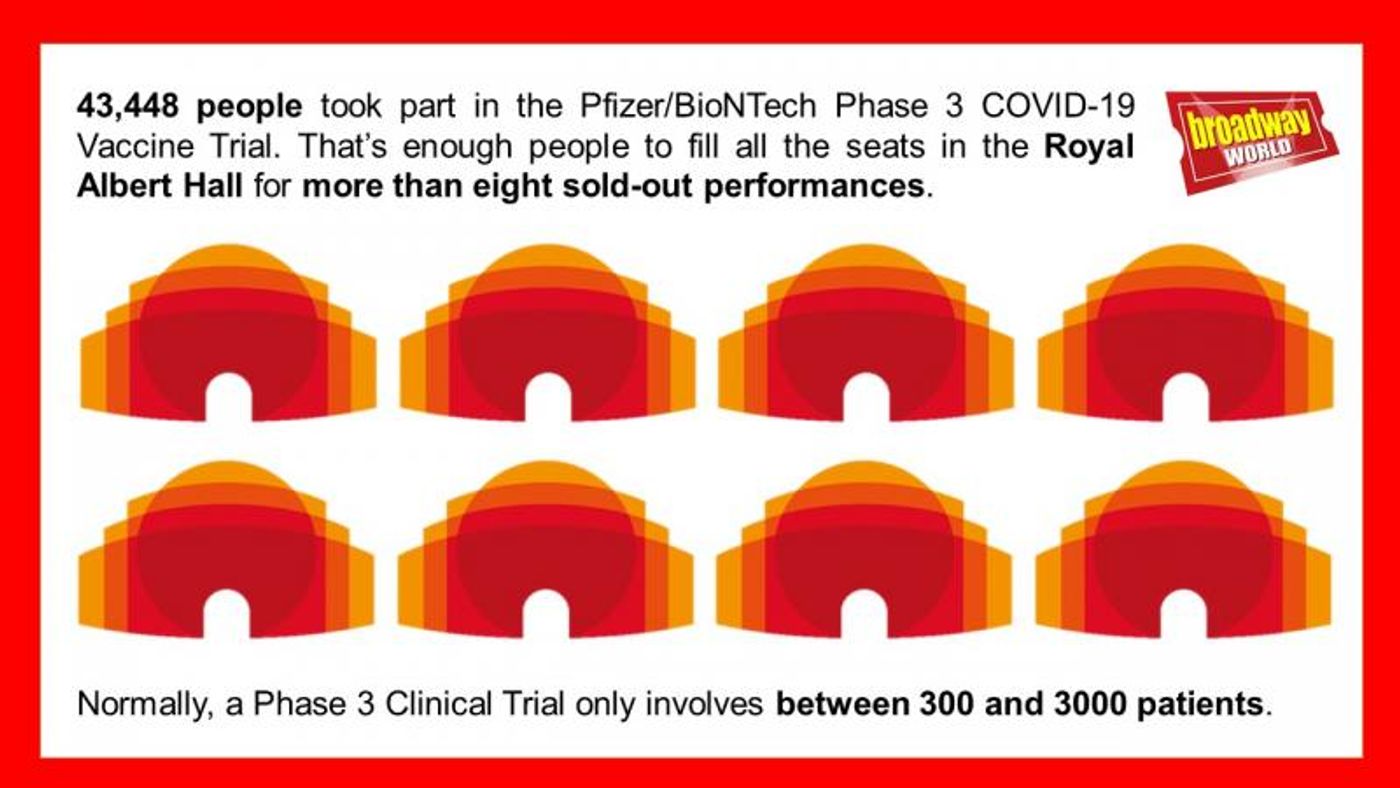
43,448 people volunteered to be part of the Pfizer/BioNTech Phase 3 trial. Half of them received the vaccine while the other half received a placebo designed to have zero effect on the body.
The capacity of the Royal Albert Hall is 5,272 seats. That means the number of people who took part in the Phase 3 Pfizer/BioNTech vaccine trial could fill the Royal Albert Hall more than eight times over.
This is far more than the usual maximum of 3000 patients involved in a Phase III trial. Bigger numbers of patients give scientists more robust evidence.
When does a clinical trial end?
A theatre show might set a limited run or announce a closure when ticket sales fall below a particular level. In the same way, people who work in clinical trials have to set a defined "endpoint", a specific time or set of events, after which they will end the trial. Pfizer/BioNTech said they would stop their Phase 3 trial when 170 patients had been diagnosed with COVID-19.
They reached that point on 9 October, so the trial "closed", or rather the scientists decided to report their findings so far. The reason they got there so quickly was that so many people volunteered to be part of the trial.
Trying to find people to be part of your clinical trial usually causes big delays in the development of a medicine. This was not an issue here - a spot on this clinical trial was a hot ticket!
Eight people out of those 170 patients diagnosed with COVID-19 had been given the Pfizer/BioNTech COVID-19 vaccine. The other 162 people who developed COVID-19 had received the placebo. From these cases so far, it appears this vaccine protects around 19 in 20 people from getting COVID-19 from what we know so far.
Pfizer/BioNTech Vaccine Safety vs the O2
Safety is an important issue to think about with new medicines. There are very strict rules that state all side effects have to be reported in a clinical trial, regardless of whether they've been caused by a drug or not.
It's a bit like having to report an audience member taking ill during a show. Joe Bloggs may have fainted during a performance of Les Misérables: The Staged Concert, but it's unlikely that Alfie Boe was to blame. The team at Les Misérables will still take note of the incident either way for their records.

Around 1 in 4 people of the 21,720 people who received the Pfizer/BioNTech vaccine experienced some sort of side effect of any kind - mild, moderate or severe. When Pfizer/BioNTech scientists looked closer at the cause of those side effects, only 4 of the serious adverse events were thought to be linked to their vaccine.
That's like 4 people in a packed O2 (seating 20,000 people) - with an additional 1720 people watching on a screen outside, suffering a serious side effect from the vaccine.
You'd have to be incredibly unlucky to be one of those people. The chances of not experiencing serious side effects and getting COVID far outweigh the chances of the Pfizer/BioNTech vaccine causing harm.
Moderna and Oxford/AstraZeneca Vaccines vs London's largest theatres
Finally, I will very briefly touch on the reported number of patients in the Phase 3 clinical trials designed to test COVID-19 vaccines made by Moderna, University of Oxford and AstraZeneca.
The Moderna Phase 3 trial results say the trial involved 30,351 volunteers. Around half received Moderna's COVID-19 vaccine while the other half received the placebo. That's enough people to fill the 2286 seats of the London Palladium for at least thirteen sold-out performances.

The University of Oxford and AstraZeneca clinical trials are a little bit more complex in their design and haven't been fully analysed, yet. The published results of their Phase 3 trial so far state that 23,848 people were involved in the clinical study.
London's largest theatre is the London Coliseum, with a capacity of 2359 seats. If everyone involved in this trial bought a ticket to a show at the Coliseum, the producers could run at least ten sold-out performances.
Final Thoughts
I hope using some of our beloved London venues to help visualise the number of people involved in these clinical trials is reassuring. If Sir Ian McKellen is willing to take a dose, it's definitely worth taking this very informed chance of protecting ourselves and others from COVID-19.
The sheer number of people who volunteered to be part of these trials, plus having a lot of money thrown at the task, have helped scientists to get to the point where they can carry out clinical trials a lot quicker than usual.
It's still early days to know for sure if the vaccine protects people from developing symptomatic COVID-19 and also passing it on, but some initial findings in the Moderna Phase 3 data look promising. If that turns out to be true, getting vaccinated would be an obvious way to feel safer congregating in a large theatre enjoying a show.
Has this information in this format been helpful? If so, please share this article with others so we can reduce vaccine hesitancy and get back into our theatres!
Photo credits: Jeff Moore, Craig Sugden, The O2, London Coliseum,
Royal Albert Hall logo used with permission
Videos

